Place the Following Substances in Order of Increasing Boiling Point
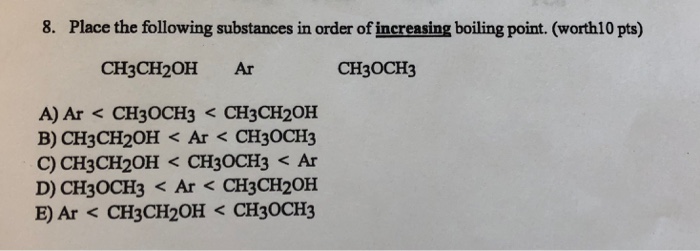
Place the following substances in order of increasing boiling point. Ar CH3OCH3 CH3CH2OH B.

Solved Place The Following Substances In Order Of Increasing Chegg Com
Ne Cl2 O2 A O2 Cl2 Ne B Ne Cl2 O2 C Cl2 Ne O2 D Ne O2 Cl2 E Cl2 O2 Ne.

. CH3CH2OH CH3OCH3 Ar D. CH 3 CH 2 OH Ar CH 3 OCH 3. A A liquid B solid C gas D critical point B A gas B solid C liquid D triple point C A gas B liquid C solid D critical point D A solid B gas C liquid D supercritical fluid E A liquid B gas C solid D triple point 14 Consider the phase diagram below.
Propane C3H8 or n-butane C4H10 diethyl ether CH3CH2OCH2CH3 or 1-butanol CH3CH2CH2CH2OH sulfur dioxide SO2 or sulfur trioxide SO3 phosgene Cl2CO or formaldehyde H2CO. Highest boiling point O Lowest boiling point. Highest boiling point Lowest boiling point.
Cl2 O2 Ne d. CH4 CO. B How do the boiling points vary through this series.
181 kg Place the following substances in order of decreasing vapor pressure at a given temperature. 21 Place the following substances in order of increasing boiling point. He Br2 F2 Group of answer choices Br2.
20 Place the following substances in order of increasing boiling point. N2 I2 Ne. Ne O2 Cl2 e.
B How do the boiling points vary through this series. CH 4 CBr 4 CH 2 Cl 2 CH 3 Cl CHBr 3 and CH 2 Br 2. View the full answer.
A Place the following substances in order of increasing. C Explain your answer to part b in terms of intermolecular. Up to 24 cash back Place the following substances in order of increasing boiling point.
Place the following substances in order of increasing boiling point. CH3OCH3 Ar CH3CH2OH E. CH4 CBr4 CH2Cl2 CH3Cl CHBr3 and CH2Br2.
CH4. Ne Cl2 O2 a. How much energy is required to vaporize 487 g of dichloromethane CH2Cl2 at its boiling point if its ΔHvap is 316 kJmol.
A Place the following substances in order of increasing volatility. Based on the type or types of intermolecular forces predict the substance in each pair that has the higher boiling point. Who are the experts.
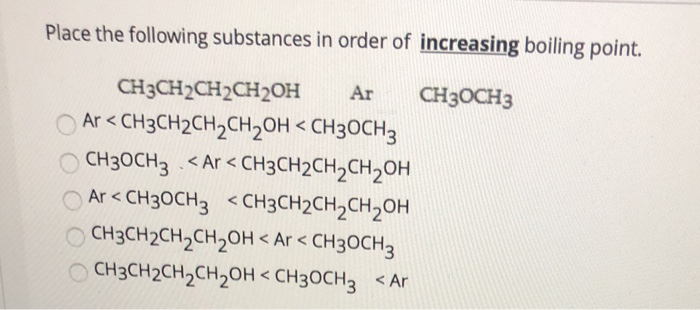
Place the following substances in order of increasing boiling point. CH3CH2CH2CH2OH Ar CH3OCH3 A Ar CH3OCH3 CH3CH2OH B CH3CH2OH Ar CH3OCH3 C CH3CH2OH CH3OCH3 Ar D CH3OCH3 Ar CH3CH2OH E Ar CH3CH2OH CH3OCH3. Experts are tested by Chegg as specialists in their subject area.
Place the following substances in order of increasing boiling point. -1958º goes first then -1915º and 100º goes last. Ne I2 N2.
CH3CH2CH3 CH3OCH3 CH3CH2OH CH3CH2CH3. Chemistry questions and answers. Cl2 Ne O2 c.
CH3CH2OH Ar CH3OCH3 C. C Explain your answer to part b in terms of intermolecular forces. Ar.
CH3CH2OH Ar CH3OCH3 CH3OCH3. O2 Cl2 Ne b. 2 on a question Place the following substances in order of increasing boiling point.
Ne Cl2 O2 a. Place the following substances in order of increasing boiling point. Ar CH3CH2OH CH3OCH3 The order of strengths of intermolecular.
O Lowest boiling point. O2 Ne Ar H2. Ne Cl2 O2 A Ne Cl2 O2 B Cl2 O2 Ne C O2 Cl2 Ne D Cl2 Ne O2 E Ne O2 Cl2 Answer.
Place the following substances in order of increasing boiling point. CH 3 CH 2 OH Ar CH 3 OCH 3 A Ar CH 3 OCH 3 CH 3 CH. I2 Ne N2.
If the dashed line at 1 atm of pressure is followed from 100 to 500C what phase changes will occur. Water or H20 starts boiling at 100ºC. Rank the following substances in order of increasing boiling point.
Arrange the following substances in order of increasing boiling point. 28 Place the following substances in order of increasing boiling point. CH 3 CH 2 OH.
Highest boiling point Lowest. Place the following substances in order of increasing boiling point. Carbon monoxide or C0 starts boiling at -1915ºC.
CH3CH2OH Ar CH3OCH3 A. Ne Cl 2 O 2 A Ne Cl 2 O 2 B Cl 2 O 2 Ne C O 2 Cl 2 Ne D Cl 2 Ne O 2 E Ne O 2 Cl 2 Answer. When we place these in order from decreasing boiling point.
Learn this topic by watching Intermolecular Forces and Physical Properties Concept Videos. Place the following substances in order of increasing boiling point. Learn this topic by watching Intermolecular Forces and Physical Properties Concept Videos.
CH 3 CH 2 OH. CO CH4. I2 N2 Ne.
CH 3 OCH 3. Click here to get an answer to your question Place the following substances in order of increasing boiling point. Ar.
Nitrogen or N2 starts boiling at -1958ºC. Ne N2 I2. Highest boiling point O Lowest boiling point.
CH3CH2OH HOCH2CH2OH CH3CH2Cl and ClCH2CH2OH Calculate the amount of heat energy in units of kilojoules required to convert 500 g of liquid ethanol CH3CH2OH at 255C to gaseous ethanol at 920C. CO. Place the following substances in order of increasing boiling point.
We review their content and use your feedback to keep the quality high. Group of answer choices.

Solved Place The Following Substances In Order Of Increasing Chegg Com
Solved 8 Place The Following Substances In Order Of Chegg Com
Solved Place The Following Substances In Order Of Increasing Chegg Com
No comments for "Place the Following Substances in Order of Increasing Boiling Point"
Post a Comment